2,5-DI(HET)ARYLPYRIDINES: SYNTHESIS BY ꞌꞌ1,2,4-TRIAZINEꞌꞌ METHODOLOGY AND PHOTOPHYSICAL PROPERTIES
Keywords:
imino ester, pyridine, 1,2,4-triazine, 1,2,4-triazine 4-oxide;, fluorescence, synthesis., aza-Diels-Alder reactionAbstract
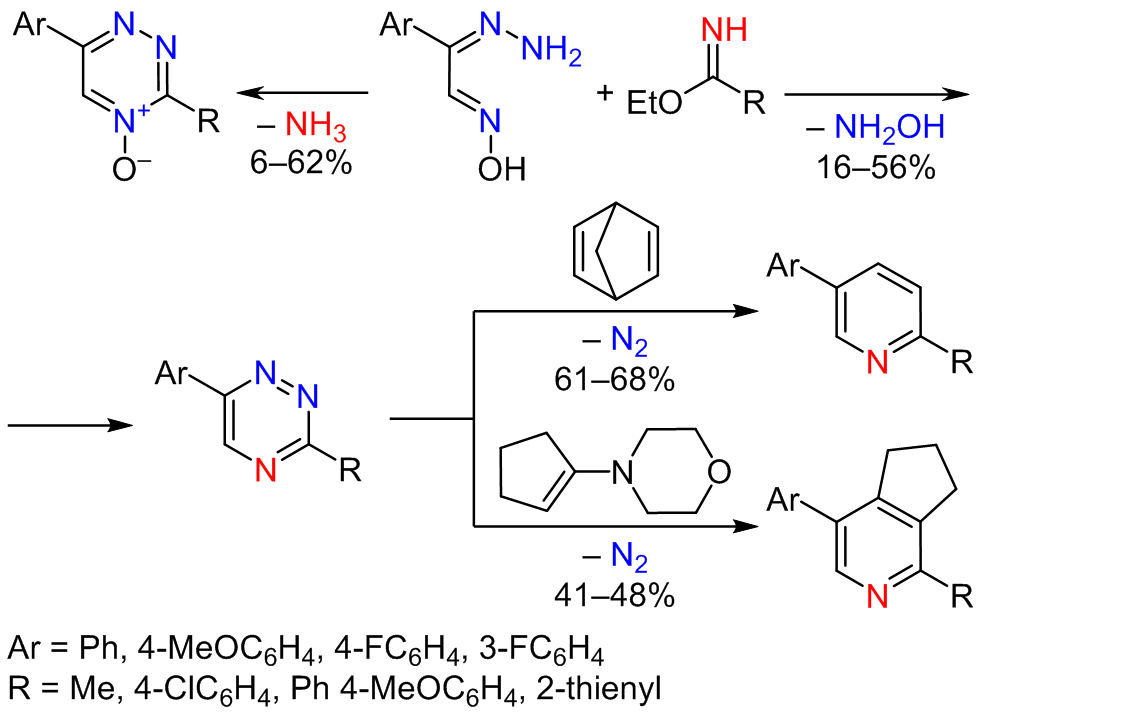
The method for the preparation of 3,6-disubstituted 1,2,4-triazines based on condensation reaction between easily available α-imino esters and isonitrosoacetophenone hydrazones was reported. The significant differences in reaction conditions, ratio of products, and yields between the developed method and the earlier reported approach were demonstrated. The corresponding 2,5-disubstituted pyridines were synthesized from the prepared 1,2,4-triazines, and their photophysical properties were studied. Studies of the photophysical properties revealed low and moderate luminescence quantum yields, and negligible solvatochromic behavior in case of 4-methoxyphenylpyridine derivative due to the role of donating methoxy group, however, with a low linearity of a Lippert–Mataga plot. Nevertheless, 2,5-disubstituted pyridines are of interest due to simple protocols of synthesis, moderate photophysical properties, and potential applicability in different scientific and industrial areas.