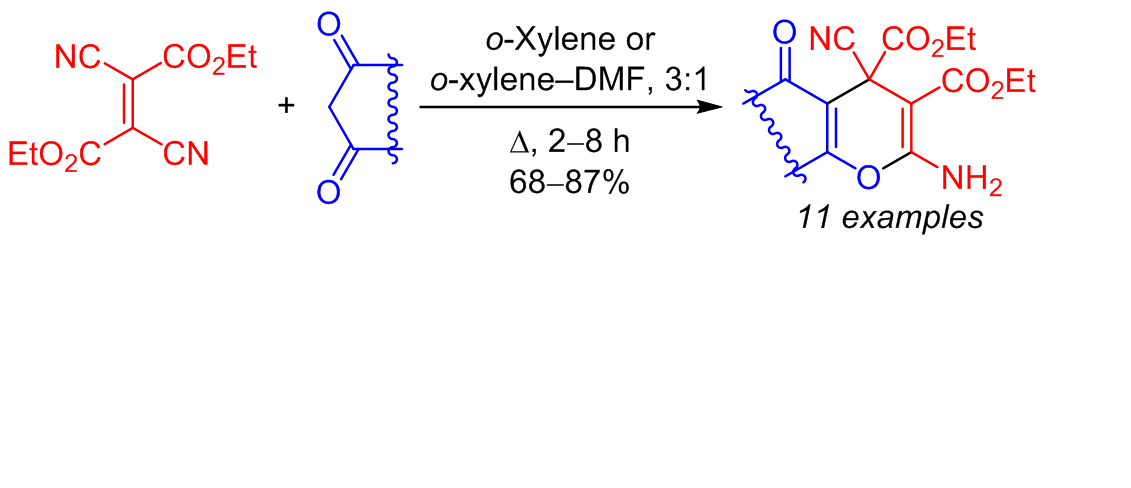
[3+3]-Аннелирование диэтилдицианофумарата и циклических 1,3-дикарбонильных соединений: cинтез конденсированных диэтил-2-амино-4-циано-4<i>Н</i>-пиран-3,4-дикарбоксилатов
Ключевые слова:
диэтил-2-амино-4-циано-4Н-пиран-3,4-дикарбоксилаты, диэтилдицианофумарат, циклические 1,3-дикарбонильные соединения, реакция Михаэля, циклизация по Торпу–ЦиглеруАннотация
При взаимодействии диэтилдицианофумарата с 1,3-дикарбонильными соединениями карбо- и гетероциклического ряда протекает [3+3]-аннелирование с образованием конденсированных диэтил-2-амино-4-циано-4Н-пиран-3,4-дикарбоксилатов. Каскадное превращение включает присоединение по Михаэлю енольной формы СН-кислоты к двойной связи диэтилдицианофумарата и последующую циклизацию по Торпу–Циглеру.