Stepwise, biradical nature of the [3+2] cycloaddition reaction between 4-nitrobenzonitrile <i>N</i>-oxide and simple ethene: a reexamination
Keywords:
nitrile N-oxides, [3+2] cycloaddition, mechanism, Molecular Electron Density TheoryAbstract
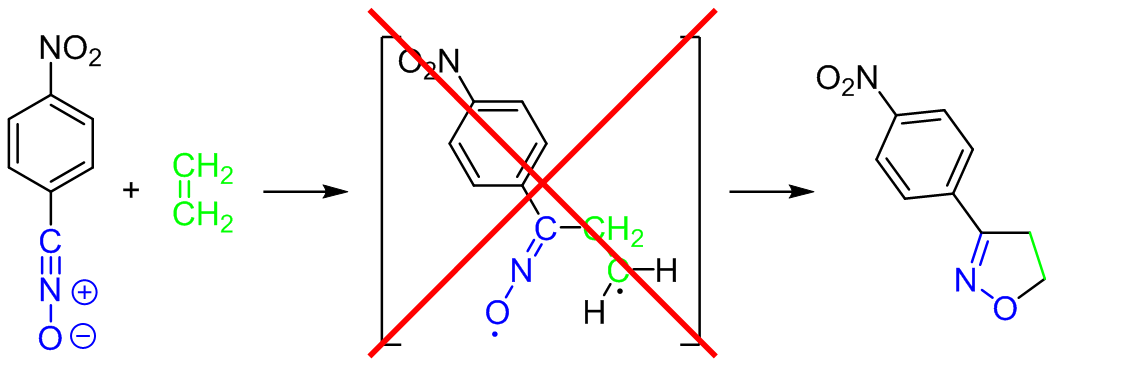
The molecular mechanism of the [3+2] cycloaddition reaction between 4-nitrobenzonitrile N-oxide and ethene was examined on the basis of the results of the wb97xd/6-311+G(d) DFT calculations. It was found, that contrary to earlier postulations, the title reaction proceeds not via a stepwise, but via a single-step mechanism. All attempts for the optimization of biradicals within this molecular segment were unsuccessful.
Downloads
Published
2025-02-13
Issue
Section
Short Communications
