SYNTHESIS AND REACTIONS OF NOVEL IMIDAZO[4,5-<i>]</i>PYRIDINE BUILDING BLOCKS
Ключевые слова:
imidazopyridines, purine bioisosteres, building blocks, coupling reactions, cyclizationАннотация
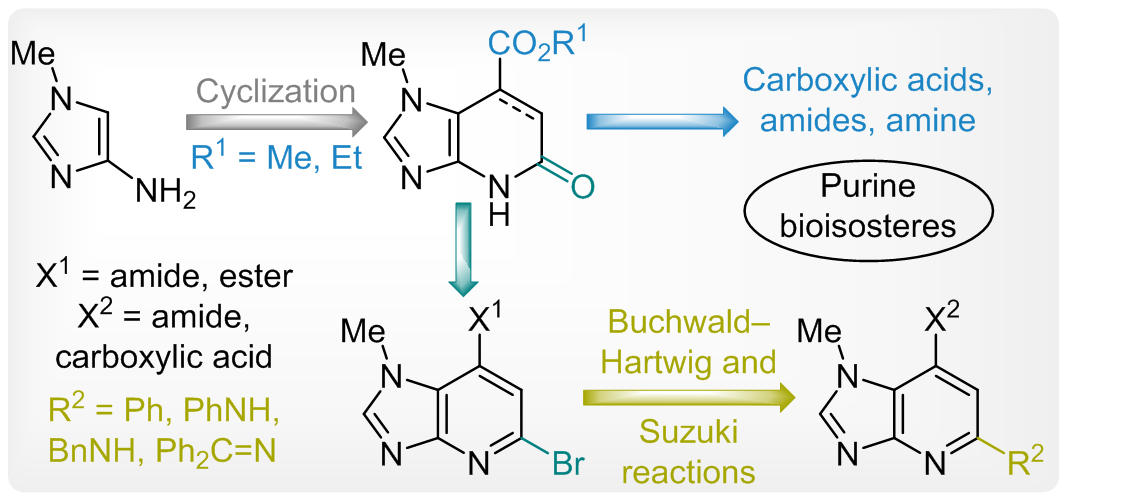
The synthesis of a novel imidazo[4,5-b]pyridine and its partially saturated derivative relied on Michael addition of 1-methyl-1H-imidazol-4-amine to fumaric/maleic or acetylene dicarboxylates, followed by intramolecular cyclization into target compounds. The common transformations of the resulting pyridine carboxylate were performed to obtain a series of building blocks, i.e. carboxylic acids, amides, and amines. The pyridone fragment was transformed into the fused bromopyridine moiety which was used for Buchwald–Hartwig and Suzuki cross-coupling reactions providing a versatile access to an extended scope of imidazopyridines. All synthesized building blocks could be considered as promising purine bioisosteres for the synthetic and medicinal chemistry.